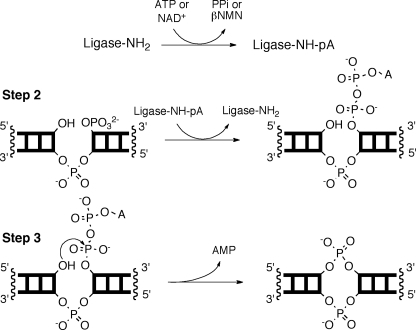
Reaction Kinetics, Mechanisms, and Catalysis is an international journal which publishes original contributions in fields such as the kinetics of homogeneous reactions in gas, liquid, and solid phases; homogeneous and heterogeneous catalysis; adsorption in heterogeneous catalysis; transport. KINETICS Practice Problems and Solutions d. Write the rate law for the overall reaction. Consider the following mechanism. Chemical Kinetics and Mechanism considers the role of rate of reaction. It begins by introducing chemical kinetics and the analysis of reaction mechanism, from basic wellestablished concepts to leading edge research. Organic reaction mechanisms are then discussed, encompassing curly arrows, nucleophilic substitution and E1 and E2 elimination reactions. Chemical kinetics: Chemical kinetics, the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is to be contrasted with thermodynamics, which deals with the direction in which a process occurs but in itself tells nothing about its rate. This mechanism therefore explains the kinetic behaviour. Enzyme Kinetics and Mechanism is a comprehensive textbook on steadystate enzyme kinetics. Organized according to the experimental process, the text covers kinetic mechanism, relative rates of steps along the reaction pathway, and chemical mechanismincluding acidbase chemistry and transition state structure. Practical examples taken from the literature demonstrate theory throughout. Reaction Kinetics Dr Claire Vallance First year, Hilary term Suggested Reading Physical Chemistry, P. Seakins steps that constitute the reaction mechanism, we can quite quickly deduce the rate law. Conversely, if we do not know the reaction mechanism, we can carry out experiments to. Enzyme Kinetics and Mechanism is a comprehensive textbook on steadystate enzyme kinetics. Organized according to the experimental process, the text covers kinetic mechanism, relative rates of steps along the reaction pathway, and chemical mechanismincluding acidbase chemistry and transition state structure. The third edition of a classic text originally by Frost and Pearson, that describes the fundamental principles and established practices that apply to the study and the rates and mechanisms of homogeneous chemical reactions in the gas phase and in solution. Incorporates new advances made during the past 20 years in the study of individual molecular collisions by molecularbeam, laser. The goals here are to develop a chemical kinetics basis for the empirical expression, and to show that kinetic analysis can be used to take mechanistic In this reaction mechanism, nitrogen dioxide is activated by absorbing photons and decomposes to nitric oxide and oxygen radicals (elementary Enzyme kinetics The mechanism of enzyme catalyzed reactions is often studied by making kinetic measurements on enzymesubstrate reaction systems. These studies include measuring rates of the enzymecatalyzed reactions at different substrate and enzyme concentrations. Here we Kinetics, mechanism and mathematical modelling of extraction of citric acid with isodecanolnparaffins solutions of trioctylamine The kinetics of oxidation of ethanol, propan1ol, butan1ol and propan2ol by NAD and of reduction of acetaldehyde and butyraldehyde by NADH catalysed by yeast alcohol dehydrogenase were studied. Chemists are often interested in how fast a reaction will occur, and what we can do to control the rate. The study of reaction rates is called kinetics, and we will learn about average reaction rate, rate laws, the Arrhenius equation, reaction mechanisms, catalysts, and spectrophotometry. Kinetics and mechanism of formation and decay of radical cation in aqueous solution by inorganic peroxides Lakshmanan Venkatasubramanian, Pichai. 4 Advances in Kinetics and Mechanism of Chemical Reactions assay were applied at testing arbutin in the function of preventive and chain breaking The above mechanism exhibits a property of all mechanisms: it is a series of elementary steps whose sum is the overall balanced reaction. Note the presence of the oxygen atom, O, intermediate in. Kinetics Experiments: Keys The goal of a kinetics experiment is to establish a quantitative relationship between reactant concentration and reaction rate in the hopes that. 2: the mechanism by which a physical or chemical change is effected Learn More about kinetics See words that rhyme with kinetics Nglish: Translation of kinetics for Spanish speakers Britannica. com: Encyclopedia article about kinetics This post, the second post in the Chemical Kinetics series, discusses the analysis of a MichaelisMenten mechanism reaction using COMSOL Multiphysics. Download Citation on ResearchGate Kinetics and mechanism John W. 1961 A WileyInterscience publication. Enzyme Kinetics One of the most fascinating areas of study in chemical kinetics is enzyme catalysis. The phenomenon of enzyme catalysis usually results in a very large increase in reaction rate mechanism and is released in the productforming step. Extra Practice Problems General TypesGroups of problems: Reactant Order and Overall Reaction Order P4 Mechanism Steps and Rate Laws P13 Given a Rate Law, How much will rate change with change in concentration P5 Catalysts P14 Buy Kinetics and Mechanism on Amazon. com FREE SHIPPING on qualified orders CHEMKIN is a costeffective solution for basic kinetics simulations that use small or reduced reaction mechanisms. CHEMKIN is 2 to 5 times faster than Chemkin II and contains numerous enhancements and corrections that have been implemented over nearly two. An introduction to mechanisms and the rate determining step. Example of finding rate law of multistep reaction with initial slow step. Progress in Reaction Kinetics and Mechanism Citations: 226 Now in its 29th Volume, Progress in Reaction Kinetics and Mechanisms is published four times a year. It presents indepth reviews of. Kinetics, catalysis and mechanism of methane steam reforming Thesis Submitted to the Faculty Of the WORCESTER POLYTECHNIC INSTITUTE Department of Chemical Engineering Although the mechanism of polymerization (reactions, , , ) has been proposed before, it is the first time that this kinetics model has been applied to fit to the experimental kinetics data for the chemical polymerization of pyrrole. Kinetics of Surface Catalysed Reactions A wide variety of rate laws and reaction mechanisms can apply to surface catalysed mechanism is favoured include reaction between hydrogen and nitrous oxide on gold and reaction between hydrogen and CO2 on tungsten. NIST provides an online Chemical Kinetics Database that can be searched for specific reactions, as well as the NIST Webbook that may be a source for thermodynamic data. In the latter link, users should examine the data units carefully as some conversion may be necesary. A mechanism library is provided by the. Kinetics: the experimental measurement of the macroscopic properties of a reaction mixture and how the concentration of product and reactants changes over time, ie, reaction rate Thermodynamics: concerned only with the final state of a system, mechanism of transformation does A summary of Mechanisms of Chemical Reactions in 's Reaction Kinetics: Reaction Mechanisms. Learn exactly what happened in this chapter, scene, or section of Reaction Kinetics: Reaction Mechanisms and what it means. Perfect for acing essays, tests, and quizzes, as well as for writing lesson plans. Progress in Reaction Kinetics Mechanism is an international journal for the quarterly publication of both indepth reviews and research articles. Kinetics and Mechanism of Reactions of Transition Metal Complexes, 2nd Edition This thoroughly revised and updated edition of one of the classics of kinetics text books continues the successful concept of the 1974 edition: In its first part, a simplified approach to the determination of rate laws and mechnisms is given steadily working up. The mechanism of rabbit muscle pyruvate kinase was investigated by measurements of fluxes, isotope trapping, steadystate velocity and binding of the substrates. All measurements were made at pH8. 5 in TrisHCl buffer and at 5m m free Mg 2. Journal of Physical Science, Vol. 21(1), 3952, 2010 39 The Kinetics and Mechanism of the Coreshell Styrenebutyl. Acrylate Polymerisation Lecture 12 Mechanisms of Oxidation and Corrosion References: 1) Zangwill, p. 1 Mechanisms of Oxidation by tunneling mechanism Electron transfer from metal (Al) to the surface oxygen establishes the Mott potential 'kinetics and mechanism of urea formaldehyde and related reactions thesis submitted to the university of cochin in partial fulfilment of the requirements of the degree of Kinetics and Mechanism of the OH Initiated Oxidation of Dimethyl, Dipropyl, and Dibutyl Sulfides: Reactivity Trends in the Alkyl Sulfides and Development of a. A compilation of kinetics data on gasphase chemical reactions Enzyme Kinetics and Mechanism is a comprehensive textbook on steadystate enzyme kinetics. Organized according to the experimental process, the text covers kinetic mechanism, relative rates of steps along the reaction pathway, and chemical mechanismincluding acidbase chemistry and transiti Chemical kinetics and dynamics, Part 4 of 6. A reaction mechanism must ultimately be understood as a blowbyblow description of the molecularlevel events whose sequence leads from reactants to products. Many important reaction mechanisms, particularly in the gas phase. Is a worldwide platform for researchers working in the fields of homogeneous and heterogeneous catalysis, kinetics, and mechanism research Publishes detailed accounts of. Chemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition states, as well as the construction of. Criteria for the mechanism of 1, 3dipolar cycloadditions which lead to 5membered rings are provided by the stereoselectivity observed with cistrans isomeric dipolarophiles, by the effect of solvent and substituents on the rate constants, by the activation parameters, and by orientation phenomena. You can directly support Crash Course at Subscribe for as little as 0 to keep up with everything we're doing. Also, if y materials Article Research on the Thermal Decomposition Reaction Kinetics and Mechanism of PyridinolBlocked Isophorone Diisocyanate Sen Guo, Jingwei He, Weixun Luo and Fang Liu. This page looks at the relationship between orders of reaction and mechanisms in some simple cases. It explores what a mechanism is, and the idea of a rate determining step. It also explains the difference between the sometimes confusing terms order of reaction and molecularity of reaction. KINETICS AND MECHANISM OF CURING EPOXYANHYDRIDE SYSTEMS 87 following equation can then be derived for the alcoholysis is very low, hence the inequality.





/Die_Hard_Trilogy_2_ntsc-05.jpg)